Welcome to "The Ostwald Process!"
People sometimes wonder what Le Châtelier's Principle is. It states; if a change is imposed on a system at equilibrium, the position of equilibrium will shift in the direction that reduces the impact of that change.
This can happen in one of four simple ways;
1. Increase the rate of Forward Reaction will cause the system to shift to the right to reach a new equilibium.
2. Increase the rate of the Reverse Reaction will cause the system to shift to the left to reach a new equilibrium.
3. Decrease the rate of the Forward Reaction will cause the system to shift to the left to reach a new equilibrium.
4. Decrease the rate of the Reverse Reaction will cause the system to shift to the Right to reach a new equilibrium.
Well that is enough of that. Onto the fun stuff; Ostwald Process!
It is the awesome method of synthesizing nitric acid from the acidation of ammonia by catalysis. Sounds tough?
Well no worries! It isn't a complicated one step equation with fifty numbers... it actually takes place in three separate equations! More equations, sure. But not too hard.
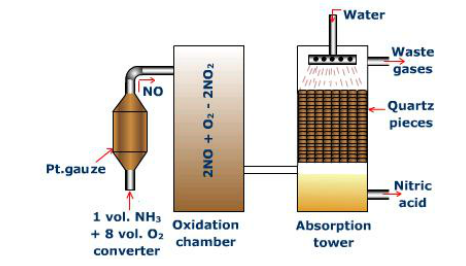
Steps
1: Mixture of one part ammonia (NH3) gas and 10 parts air
•Fed into catalytic
•1292 oF to 1472 oF (700 oC -800 oC)
•Using platinum catalyst
•Ammonia combines with Oxygen (O2)
•Produces Nitric Oxide
(Balanced Equation) •(NO): 4NH3 + 5O2 à 4NO + 6H2O
2: In oxidation chamber
•122 oF (50 oC)
•Nitric Oxide is combined with Oxygen
•Produces Nitrogen Dioxide
(Balanced Equation) •2NO + O2 à NO2
3: In absorption chamber
•Nitrogen Dioxide is dissolved in water
•Given Nitric Acid (HNO3) and Nitric Oxide
•Can be recycled
(Balanced Equation) •3NO2 + H2O à 2HNO3 + NO
About the Process
The Ostwald Process is exothermic; meaning it releases energy. It also favors the "forward, shift right" reaction. So it decreases the temperature and increases the concentration, pressure, and volume.
Is that it? Oh no It is not!
Knowing that each step is exothermic, increasing the temperature anywhere along the line will shift the equilibirum to the left, reducing the yeild; according to Le Châtelier's Principle.